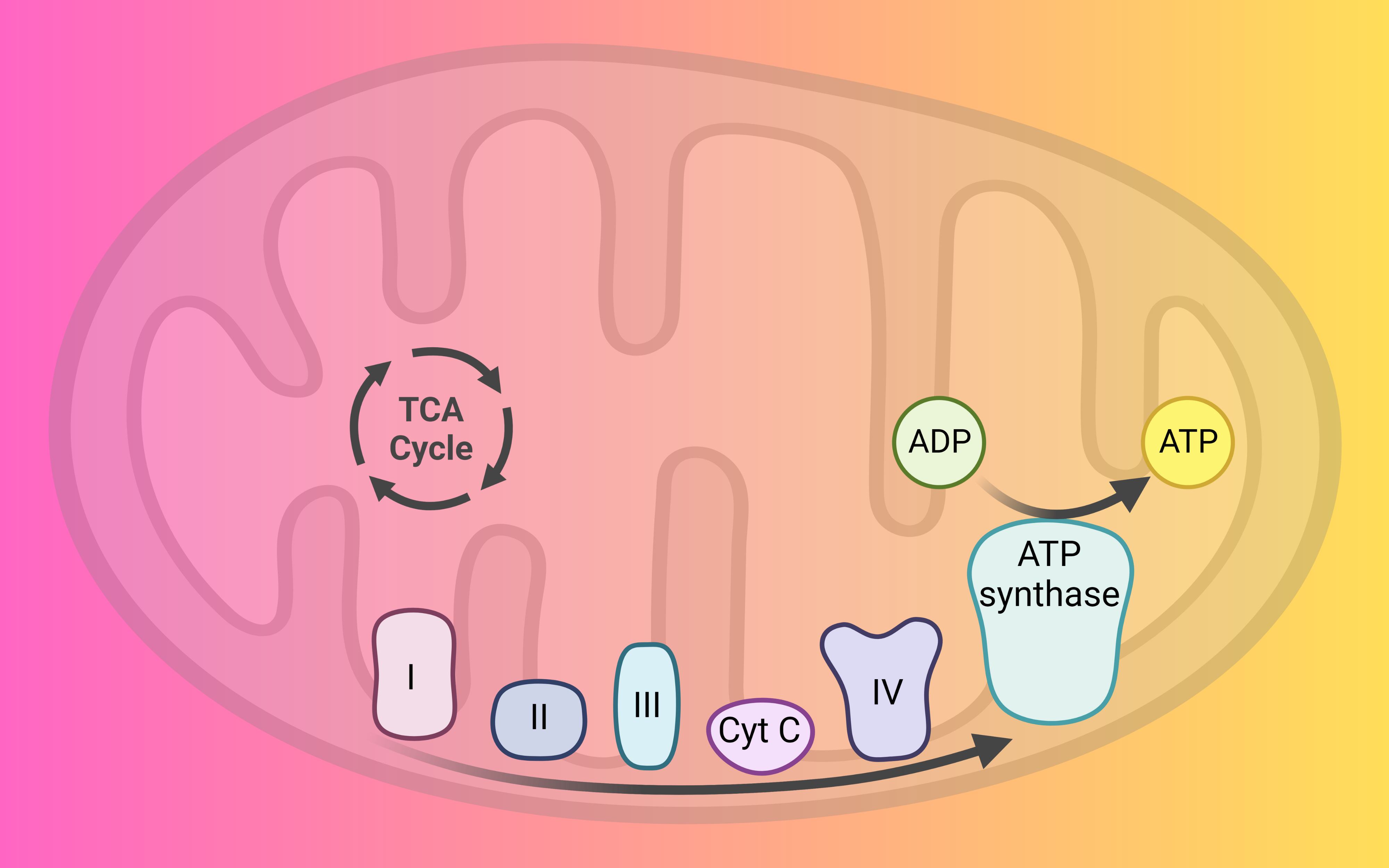
The respiratory chain, also known as the electron transport chain, is a series of protein complexes located in the inner mitochondrial membrane of eukaryotic cells and the plasma membrane of prokaryotic cells. The respiratory chain is responsible for generating the majority of ATP (adenosine triphosphate) in aerobic organisms through the process of oxidative phosphorylation.
It is the final stage of cellular respiration. It takes place in the inner mitochondrial membrane of the cell and is responsible for the conversion of the energy carried by NADH and FADH2 into ATP. The ETC is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process.
The ETC is a series of protein complexes that work together to transfer electrons from NADH and FADH2 to oxygen, the final electron acceptor. These electrons move from complex to complex, each one being more electronegative than the previous one, releasing energy in the process. The energy released by these reactions is used to pump protons across the inner mitochondrial membrane, creating a proton gradient. This proton gradient is used by the enzyme ATP synthase to generate ATP from ADP and inorganic phosphate.
It’s worth noting that the ETC is not only a fundamental process in cellular respiration but also plays an important role in the metabolism of many organisms. It’s also responsible for the production of ATP in the absence of oxygen, such as in anaerobic respiration.
In summary, the electron transport chain (ETC) is the final stage of cellular respiration that takes place in the inner mitochondrial membrane of the cell. It’s responsible for the conversion of the energy carried by NADH and FADH2 into ATP. The ETC is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process. It’s a fundamental process in cellular respiration and also plays an important role in the metabolism of many organisms.
Table of Contents
Location of the electron transport chain
The electron transport chain (ETC) takes place in the inner mitochondrial membrane of the cell. The mitochondria are organelles that are often referred to as the “powerhouses” of the cell because they generate the majority of the cell’s ATP through cellular respiration.
Mitochondria are double-membraned organelles, with an outer membrane and an inner membrane. The ETC takes place in the inner mitochondrial membrane, which is the space between the outer membrane and the inner membrane. The protein complexes and electron carriers that make up the ETC are embedded in the inner mitochondrial membrane. The ETC is dependent on the presence of cofactors such as NAD and FAD which are also located in the mitochondrial matrix.
It’s worth noting that the ETC is not limited to one organelle or compartment in the cell. Some of the enzymes involved in the ETC are located in the mitochondria, while others are located in the cytoplasm.
Overall, the electron transport chain (ETC) is a metabolic process that takes place in the inner mitochondrial membrane of the cell. The ETC is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process. This energy is used to pump protons across the inner mitochondrial membrane, creating a proton gradient that is used by the enzyme ATP synthase to generate ATP from ADP and inorganic phosphate. The ETC is a fundamental process in cellular respiration and also plays an important role in the metabolism of many organisms.
Steps of the electron transport chain process

The electron transport chain (ETC) is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process. The ETC can be divided into the following steps:
1. Electrons are transferred from NADH or FADH2 to Complex I (NADH dehydrogenase or succinate dehydrogenase) by the enzyme NADH dehydrogenase or succinate dehydrogenase. Complex I also pumps protons across the inner mitochondrial membrane, creating a proton gradient.
2. Electrons are then transferred from Complex I to Complex III (cytochrome bc1 complex) by the electron carrier Coenzyme Q. Complex III also pumps protons across the inner mitochondrial membrane.
3. Electrons are then transferred from Complex III to Complex IV (cytochrome c oxidase) by the electron carrier cytochrome c. Complex IV also pumps protons across the inner mitochondrial membrane.
4. Electrons are then transferred from Complex IV to oxygen, the final electron acceptor, by the enzyme cytochrome c oxidase. Oxygen is reduced to water in this process.
5. The energy released by the movement of electrons through the ETC is used to pump protons across the inner mitochondrial membrane, creating a proton gradient.
6. The proton gradient is used by the enzyme ATP synthase to generate ATP from ADP and inorganic phosphate.
It’s worth noting that the ETC is a highly regulated process, controlled by allosteric enzymes, hormones, and other signaling molecules. This regulation ensures that electrons are transferred in a controlled manner, avoiding metabolic imbalances and providing the appropriate amount of energy for the cell’s needs.
In summary, the electron transport chain (ETC) is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process. The ETC is responsible for the conversion of the energy carried by NADH and FADH2 into ATP. It’s a highly regulated process, controlled by allosteric enzymes, hormones, and other signaling molecules. It’s a fundamental process in cellular respiration and also plays an important role in the metabolism of many organisms.
It’s also worth noting that the ETC is not only a source of energy production, but also plays a key role in maintaining the balance of redox potential in the cell. The ETC is responsible for the transfer of electrons from NADH and FADH2 to oxygen, the final electron acceptor. This transfer of electrons helps to maintain the balance of redox potential in the cell, ensuring that the cell can maintain the appropriate level of energy production and metabolism.
It’s also important to mention that the ETC is a highly complex process, involving multiple protein complexes and electron carriers. Each complex and carrier plays a unique role in the transfer of electrons, and any disruptions or mutations in these complexes and carriers can have severe consequences on the cell’s energy production and metabolism.
In conclusion, the electron transport chain (ETC) is the final stage of cellular respiration that takes place in the inner mitochondrial membrane of the cell. It’s responsible for the conversion of the energy carried by NADH and FADH2 into ATP. The ETC is a series of protein complexes and electron carriers that transfer electrons through a series of oxidation-reduction reactions, releasing energy in the process. The ETC is a highly regulated process, controlled by allosteric enzymes, hormones, and other signaling molecules. It’s a fundamental process in cellular respiration and also plays an important role in the metabolism of many organisms. It’s responsible for maintaining the balance of redox potential in the cell and it’s a highly complex process involving multiple protein complexes and electron carriers. Any disruptions or mutations in these complexes and carriers can have severe consequences on the cell’s energy production and metabolism.
How energy is produced during cellular respiration

Energy is produced during cellular respiration through a series of enzyme-catalyzed reactions that convert glucose into CO2 and water, releasing energy in the process. This process is divided into three stages: glycolysis, the citric acid cycle, and the electron transport chain.
1. In the first stage, glycolysis, glucose is broken down into pyruvate. This process generates 2 ATP molecules and 2 NADH molecules as energy carriers.
2. In the second stage, the citric acid cycle, pyruvate is converted into acetyl-CoA, which is then broken down into CO2 and water. This process generates 2 ATP molecules, 6 NADH molecules and 2 FADH2 molecules as energy carriers.
3. In the final stage, the electron transport chain, the energy carried by NADH and FADH2 is used to pump protons across the inner mitochondrial membrane, creating a proton gradient. This proton gradient is then used by the enzyme ATP synthase to generate ATP from ADP and inorganic phosphate. This process generates 34 ATP molecules.
Overall, the net gain of ATP during cellular respiration is 38 ATP molecules per glucose molecule. This process is highly regulated to ensure that energy is produced in a controlled manner, avoiding metabolic imbalances and providing the appropriate amount of energy for the cell’s needs.
In summary, energy is produced during cellular respiration through a series of enzyme-catalyzed reactions that convert glucose into CO2 and water, releasing energy in the process. The process is divided into three stages: glycolysis, the citric acid cycle, and the electron transport chain. These stages work together to produce ATP, NADH, and FADH2 as energy carriers, which are used to power the cell’s metabolic processes. The net gain of ATP during cellular respiration is 38 ATP molecules per glucose molecule.
It’s also worth noting that cellular respiration is not limited to glucose as the sole source of energy. Other molecules such as fatty acids and amino acids can also be used as energy sources, and they go through a similar process of breakdown and oxidation, ultimately producing ATP.
Additionally, it’s worth mentioning that cellular respiration is not always aerobic, meaning it does not always require oxygen. In the absence of oxygen, cells can use anaerobic respiration to generate ATP through a process called fermentation. The most common types of fermentation are lactic acid fermentation and alcoholic fermentation, which are used by different organisms depending on their environment and metabolic needs.
Overall, cellular respiration is a vital process for all living organisms, providing energy for the cell’s metabolic processes. It’s not limited to glucose as the sole source of energy, and other molecules such as fatty acids and amino acids can also be used. It’s also not always aerobic, and cells can use anaerobic respiration to generate ATP through fermentation. Overall, cellular respiration is a highly regulated process that ensures that energy is produced in a controlled manner, avoiding metabolic imbalances and providing the appropriate amount of energy for the cell’s needs.